$250.00
QSAR models and Read Across strategies
Presented at the 2018 OpenTox Euro Conference in Athens, Greece.
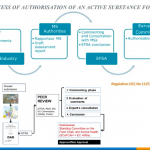
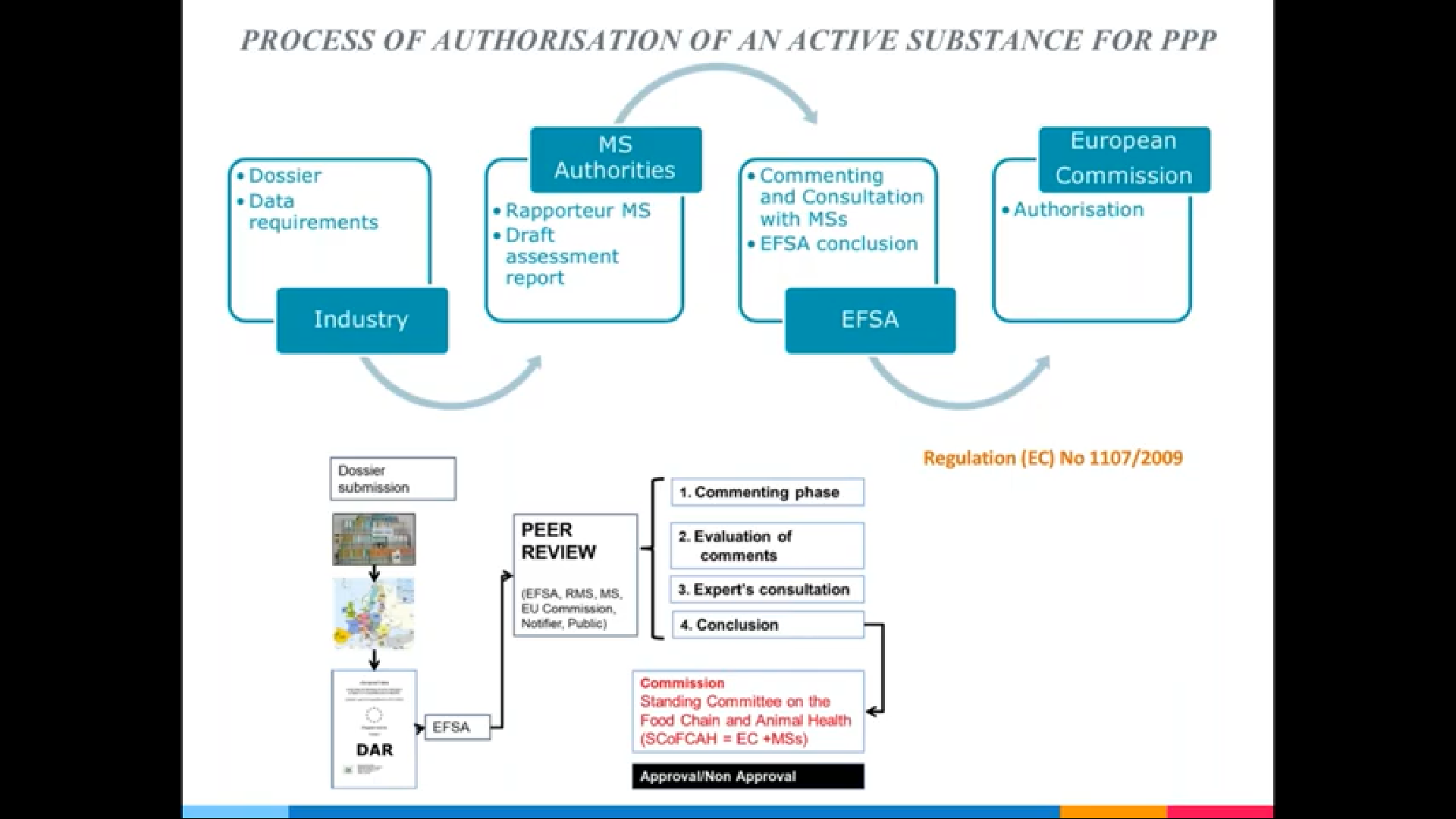
The guidance document issued by the European Food Safety Authority (EFSA) Panel on Plant Protection Products and their Residues establishes a process for the definition of pesticide residues, including the evaluation of the risk based on toxicity and potential for dietary exposure. While a comprehensive toxicological dossier is developed for active substances, often none or only limited information about toxicological properties of their metabolites is available. Thus, the use of QSAR models and read across is proposed for the assessment of genotoxic potential of all identified metabolites as a first step in the residue definition procedure.
This is the first large-scale comparison of QSARs for assays / endpoints different from bacterial gene mutation.
The overall comparisons of the sets of predictions, which pointed to strengths and weaknesses of the different QSAR predictive systems, will be presented.