Toxicological sciences are changing rapidly, with a strong trend away from animal experiments and towards new alternative methods comprising novel in vitro and in silico approaches.
Toxicity is going through a paradigm shift with the advent of new technologies, big data in biology, analytics and AI techniques together with change in the regulatory landscape.
“Since we are the first local chapter to launch, we have to show what can be accomplished and set an example for other chapters,” says Steven Edwards.
Following the successful SkinSens ITS release during the Society of Toxicology annual meeting 2017, we received plenty of positive feedback and valuable suggestions for additional features.
Our OpenTox Euro Conference 2017 was held 22-23 November at Hotel Stücki and Technology Park in Basel, Switzerland.
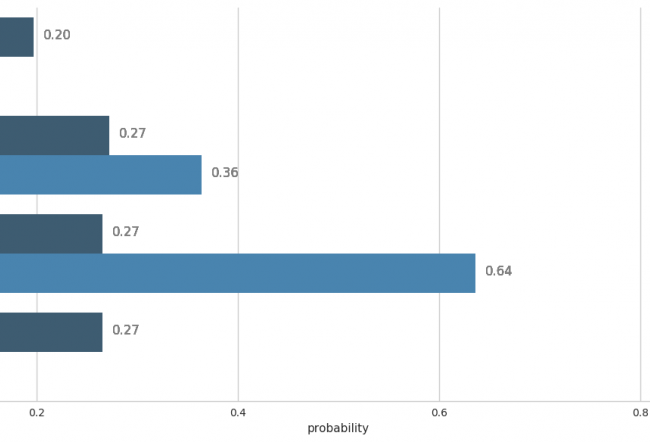
“To judge the toxicity of a compound, we must compare it with previous studies, make the data available and combine with specific compounds,” explains Chris Evelo